3D Printing Unpeeled: SprintRay Midas & BMF 5109(K)
Description
Boston Micro Fabrication (BMF) got U.S. Food and Drug Administration (FDA) 510(k) clearance for its UltraThineer micro stereolithography (PµSL) veneer solution. The company will sell the solution to dental labs making a further move into solutions and away from only selling 3D printers. The veneer is three times thinner than comparable ones and should be more comfortable to patients.
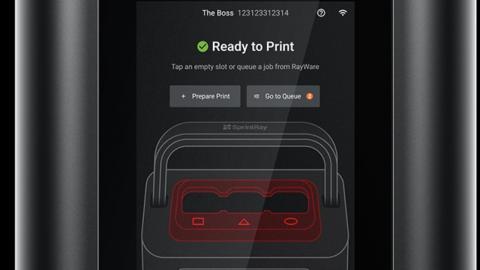
SprintRay, the end to end dental, materials, software and printers provider for dental has released the Midas Digital Press 3D Printer. This has a vacuum-sealed Resin Capsule System, which provides easier handling for more viscous resins. Resins can contain up to 70% ceramic filler. The solution shows the maturity of the 3D printing market for dental where we're seeing many solutions for many players.